Data Quantity
Quality of Analyses
Data Quantity

Quality of Analyses

Data Quantity

Quality of Analyses

Data Quantity

Quality of Analyses

The L-Matrix Method addresses key challenges met by pharmaceutical, medical technology, biotech and life science companies in the Research and Development (R&D) process, by enabling evidence-based decision making to:
The L-Matrix Method ultimately increases the success of your R&D process: by overriding intrinsic weaknesses and identifying hidden strengths, thus granting your company competitive intelligence founded on differential assessment of competitor or own compounds/products.
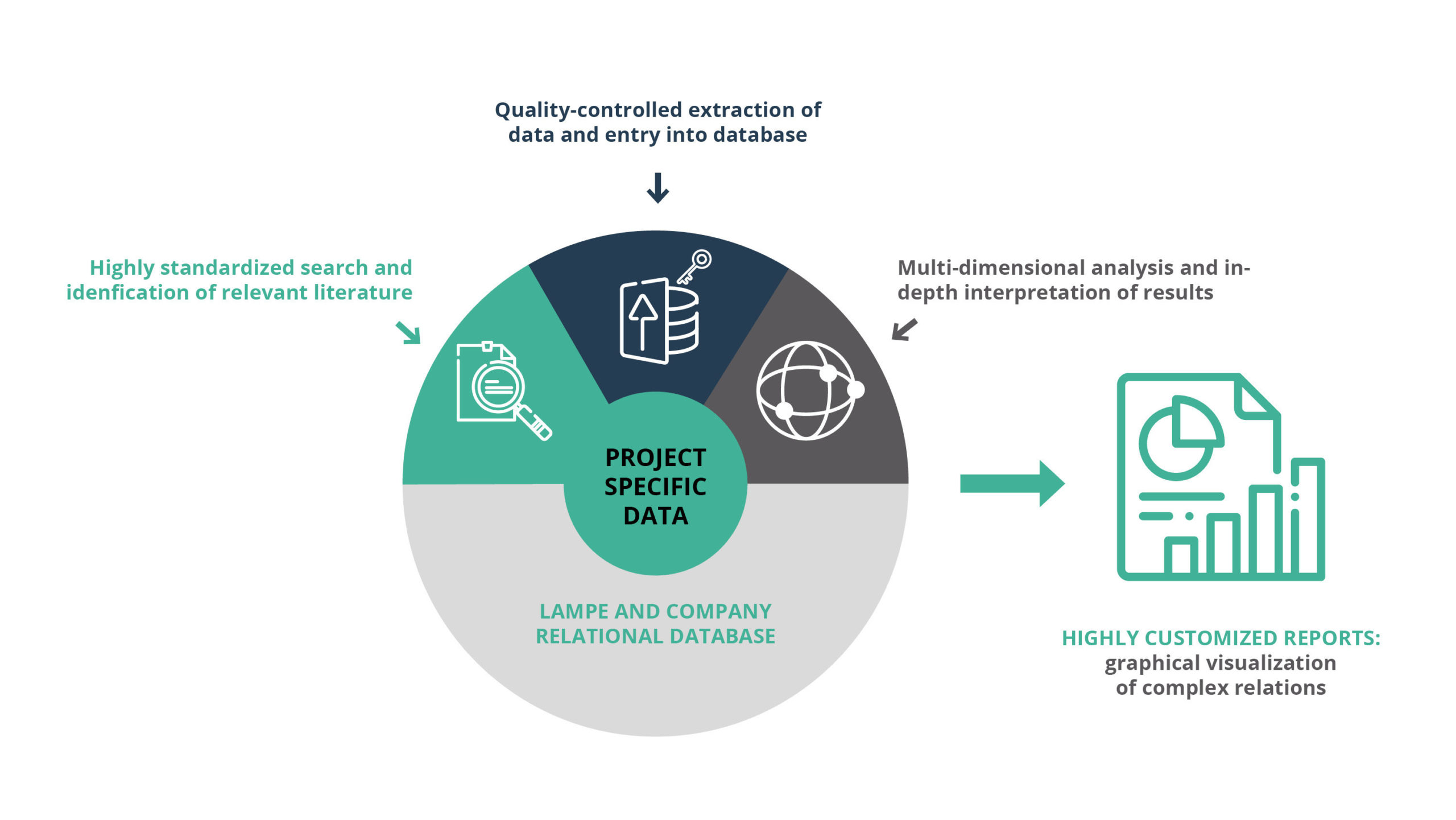
Selected by R&D professionals and digitally processed with human intellect and understanding.
Best possible data quality (SOP-driven & quality-controlled data selection and evaluation)
Complete data set, only relevant data (highest possible sensitivity and specificity of search process)
Traceability of each data point
Intuitive understanding of all aspects
360° view (all perspectives)
Ready to use for decisions (no additional efforts for further data search/compilation needed)